
Smart syringes are innovative drug delivery systems that incorporate technology to improve the administration of medications, vaccines, and clinical trials while gathering vital, secure patient data and advancing protocol adherence. With the rising prevalence of injectable biological products, the prefilled syringe (PFS) is becoming a primary mode of drug administration. Indeed, the global prefilled syringe market is projected to reach $13.33 billion by 2028, with a compound annual growth rate (CAGR) of 11.11%.
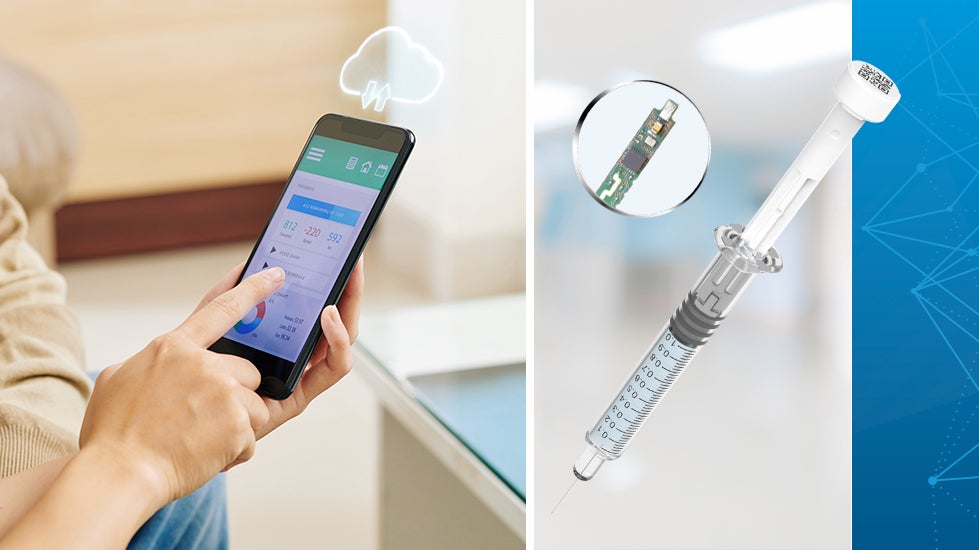
Two key applications for prefilled syringes
Prefilled syringes are being applied in two key areas: clinical trials and chronic disease therapy.
But both clinical trials and chronic disease management initiatives have had challenges with accurate administration. For clinical trials, pharma companies often have subjective patient feedback but little objective data on treatment adherence, which can compromise the outcome of the clinical trial. In chronic disease management, adherence and compliance errors are frequent. Self-injection doses are done without the supervision of a healthcare professional and without monitoring or record keeping.
Smart syringes can represent a solution for these challenges, as they offer the following advantages over conventional syringes:
- Improved dose accuracy
- Data collection and post-use analysis
- Ability to monitor patients remotely
These use cases require optimal compliance to injection protocol that is essential to treatment success.
To date, the market has provided commercially available connected pens and autoinjectors, but not syringes that incorporate sensors with connectivity to increase accuracy and drug administration compliance.
Smart syringe design requirements present challenges
Designing a smart prefilled syringe presents a range of technical challenges that must be addressed, including:
- Miniaturization: syringe size and form factor remain the same as traditional syringes
- User-centricity: user’s injection practice unchanged
- Cybersecurity: data protection and secure transmission to cloud
- Sustainability: low carbon footprint and eco-sustainable product
- Price competitiveness: low additional cost for sensor and connectivity
To address these design challenges while remaining responsive to the market, Flex has developed a smart syringe reference design platform that lowers the barrier to market while enabling companies to deliver patient benefits faster.
Benefits of a smart prefilled syringe
Healthcare provider benefits include:
- Better dose accuracy
- Greater dose tracking
Pharma and MedTech company benefits include:
- Automated data collection for clinical trials
- Unique recyclable platform
- Minimal cost impact
- Compatible design with existing syringe packages
- Faster time-to-market
The smart syringe reference design platform is part of Flex’s goal to enable pharma and MedTech companies to provide the most advanced medical devices for patient care. It provides advanced technology housed in a common syringe form factor for ease of use.
Developing a smart prefilled syringe to advance remote patient care and clinical trial outcomes
Ordinary, unconnected syringes lack complete data capture, monitoring, and transmission. In contrast, Flex’s smart syringe reference design platform has been created with advanced digital features and specifically designed to fit those features into small, volume-fill syringes, such as a 1mL prefilled syringe format.
Because the syringe’s piston incorporates digital features and can transfer data through Bluetooth low energy (BLE), administration of the drug can be easily monitored without compromising the patient’s privacy and cybersecurity. The smart syringe can accurately sense the delivered dose and transmit the injection log together with a timestamp to a clinical study portal. This allows effective injection tracking, while enabling post-use data analysis to enhance the efficacy of clinical trials.
It also addresses cybersecurity concerns with the industry-standard AES128-EAX scheme, which provides encryption to protect transmitted dose data, as well as syringe-to-cloud secure authentication.
The smart syringe reference platform is designed with sustainability in mind and is composed of eco-sustainable resin material to lower the product’s carbon footprint and environmental impact. Additionally, it can be easily disassembled at the product’s end-of-life to enable circular economy processes and reverse logistics.
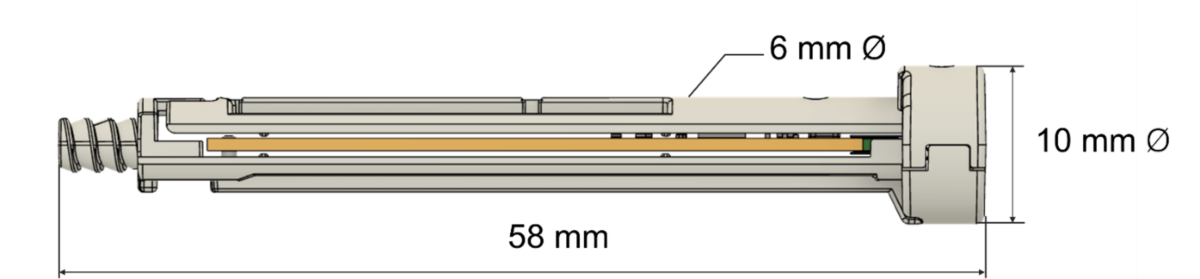
Mechanically, the Flex design has an identical length and diameter of standard rods; incorporates a circuit board and a coin-cell battery; is based on environmentally friendly Polypropylene; is compatible with Ethylene Oxide (ETO) sterilization; and is designed for high-volume injection molding.
Improved patient care and operational administration with smart syringe
Our smart syringe reference design platform offers numerous benefits to pharma and healthcare providers.
Pharma companies can now receive objective data based on real measurements when executing clinical trials to validate efficacy of new injectable-based therapies, thereby improving drug time to market and increasing revenues. Meanwhile, healthcare providers are better able to manage patient chronic disease cases, in which patients often skip injections in home-based therapy. With smart syringes, patients are more closely monitored for treatment efficacy, and the delivered dose can be tracked precisely with greater accuracy.
In summary, Flex’s smart syringe reference platform offers a range of benefits that improve patient safety, enhance healthcare provider practices, and contribute to more effective healthcare delivery. Integration of digital technology into smart syringes plays a vital role in advancing healthcare and improving patient outcomes.



